Further Differentiation within the ER
OK this week I've been obsessed with the endoplasmic reticulum (ER). This organelle is comprised of a continuous network of membranous tubes (and sheets) that extends to the cell periphery. In addition ER sheets also envelopes the nucleus - forming a bilayered nuclear envelope with an outer nuclear membrane and inner nuclear membrane.
Here's a pic of the ER from a previous post:

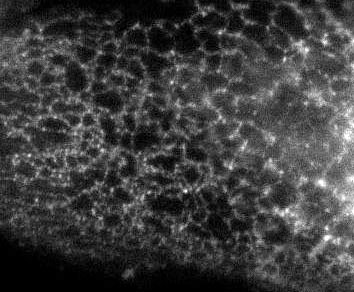
The extended part of the ER though is mostly tubular, as I displayed in an earlier post on Immunofluorescence images:
So the question becomes ... how do you form tubular organelles?
Well Gia Voeltz, from our lab just published a paper in Cell on how a class of proteins (Reticulons and DP1-related proteins) are responsible for this architecture. Reminiscent of an earlier post where I described how Nesprins are confined to the outer nuclear membrane (and thus help to differentiate that portion of the ER), reticulons and DP1 define another part of the ER, the reticular or tubular part. So reticulons are excluded from the nuclear membrane. But there's more, Gia used an assay to form tubules in vitro (i.e. in a testube) from ER vesicles derived from frog eggs. She could inhibit the tube formation by modifying reticulons or by adding antibodies against reticulons. In addition the way reticulons are inserted into the membrane is not typical of most membrane proteins, and this strange "topology" may help them to curve membranes and thus help to form tubes.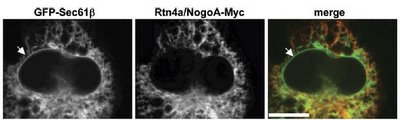
To read more check out the paper.
Ref: Gia K. Voeltz, William A. Prinz, Yoko Shibata, Julia M. Rist and Tom A. Rapoport, A Class of Membrane Proteins Shaping the Tubular Endoplasmic Reticulum Cell (2006) 124:573-586
Here's a pic of the ER from a previous post:

The extended part of the ER though is mostly tubular, as I displayed in an earlier post on Immunofluorescence images:

So the question becomes ... how do you form tubular organelles?
Well Gia Voeltz, from our lab just published a paper in Cell on how a class of proteins (Reticulons and DP1-related proteins) are responsible for this architecture. Reminiscent of an earlier post where I described how Nesprins are confined to the outer nuclear membrane (and thus help to differentiate that portion of the ER), reticulons and DP1 define another part of the ER, the reticular or tubular part. So reticulons are excluded from the nuclear membrane. But there's more, Gia used an assay to form tubules in vitro (i.e. in a testube) from ER vesicles derived from frog eggs. She could inhibit the tube formation by modifying reticulons or by adding antibodies against reticulons. In addition the way reticulons are inserted into the membrane is not typical of most membrane proteins, and this strange "topology" may help them to curve membranes and thus help to form tubes.
Here are a black & white images with a color overlay (from Gia's paper), that demonstrate the unique distribution that reticulons adopt. Notice how a general ER marker (Sec61-beta) is in the tubular portion and in the nuclear envelope, but that reticulon4a (also called NogoA) is only found in the tubular ER. Note how the black and white image of Rtn4a (but not the color overlay!) clearly demonstrates how reticulons are excluded from the nuclear envelope.

To read more check out the paper.
Ref: Gia K. Voeltz, William A. Prinz, Yoko Shibata, Julia M. Rist and Tom A. Rapoport, A Class of Membrane Proteins Shaping the Tubular Endoplasmic Reticulum Cell (2006) 124:573-586


<< Home